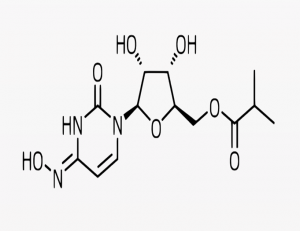
Molnupiravir
EIDD-2801/MK-4482
Molnupiravir is an antiviral pill by pharmaceutical giant Merck that aims to prevent mild to moderate cases of covid-19 from becoming severe cases that result in hospitalization or death. It has been shown to reduce the risk of death among people infected with the coronavirus.
Recently approved for emergency use authorization by the FDA, Molnupiravir is currently available, becoming the First Oral Antiviral Medicine for COVID-19. In a push to increase access to developing markets, Merck has licensed their product to eight generics manufacturers in India.

1. About Molnupiravir
Molnupiravir is an antiviral medicine that works by stopping the virus that causes coronavirus (COVID-19) from growing and spreading. It’s used to treat early COVID-19 infection and help to prevent more severe symptoms.
Key Facts
You will take 4 molnupiravir capsules twice a day for 5 days. Even if you start to feel better it’s important that you complete the course. It’s important that you start taking molnupiravir within 5 days of getting COVID-19 symptoms. Molnupiravir starts working very soon after taking it.
Common side effects of molnupiravir include feeling dizzy and headaches. If you feel dizzy, do not drive, cycle or use tools or machinery. Molnupiravir is not recommended during pregnancy. It’s important to use reliable contraception while taking molnupiravir and for 4 days after your last dose.
2. Who Can And Cannot Take Molnupiravir
WHO CAN TAKE MOLNUPIRAVIR
You may be eligible for molnupiravir if all of these apply

♦ You’re an adult aged 18 years and older
♦ You’re in the high risk group
♦ You’ve had a positive PCR test within the last 72 hours
♦ you’ve had coronavirus (COVID-19) symptoms within the last 5 days
WHO CAN'T TAKE MOLNUPIRAVIR
Molnupiravir is not suitable for some people. Tell your doctor before starting to take this medicine if:

♦ You’re pregnant, trying to get pregnant or breastfeeding
♦ You have ever had an allergic reaction to molnupiravir or any other medicine
3. What We Offer
STRAIGHT
from the manufacturer
Direct delivery optimises fast availability and control over storage space, avoiding unnecessary increased costs.
NO
intermediaries

No unauthorised handling of your products guarantees the quality and integrity of your order
FAST
delivery

No transition through third-party storages and their administrative systems simply means no delays
4. Licensed Generics / SOP
- Buyer Issues PO Only local companies with a valid WDA are authorized to purchase*
- SPA / Escrow Agreement between Buyer and Seller is signed
- Invoice is issued by Seller
- Buyer pays a 50% down payment with the remainder transferred into escrow
- Seller releases to buyer the following document
- Factory order confirmation
- Production batch numbers
- Product report
- Release of remaining funds in escrow
- Seller provides BOL and AWB
- Shipping of batch
5. Licensed Generics / Availability
- Cipla / Dr. Reddy’s / Aurobindo / SUN Pharma
- Delivery time depending on order size
- $71.01 USD / Box* CIF
6. Current Approved Countries
- Afganisthan (LIC_+LDC_+SSA)
- Algeria (LMIC)
- Bangladesh (LIC_+LDC_+SSA)
- Belize (UMIC)
- Bhutan (LIC_+LDC_+SSA)
- Bolivia (LMIC)
- Botswana (LIC_+LDC_+SSA)
- Burkino faso (LIC_+LDC_+SSA)
- Burundi (LIC_+LDC_+SSA)
- Cambodia (LIC_+LDC_+SSA)
- Central african republic (LIC_+LDC_+SSA)
- Chad (LIC_+LDC_+SSA)
- Congo (LIC_+LDC_+SSA)
- Cuba (UMIC)
- Djibouti (LIC_+LDC_+SSA)
- Dominica (UMIC)
- El salvador (LMIC)
- Equitoreal Guinea (LIC_+LDC_+SSA)
- Egypt (LMIC)
- Eritrea (LIC_+LDC_+SSA)
- Eswatini (LIC_+LDC_+SSA)
- Ethiopia (LIC_+LDC_+SSA)
- Fiji (UMIC)
- Gabon (LIC_+LDC_+SSA)
- Gambia (LIC_+LDC_+SSA)
- Ghana (LIC_+LDC_+SSA)
- Greneda (UMIC)
- Guatemala (UMIC)
- Guinea (LIC_+LDC_+SSA)
- Guinea bissau (LIC_+LDC_+SSA)
- Guyana (UMIC)
- Haiti (LIC_+LDC_+SSA)
- Honduras (LMIC)
- India (LMIC)
- Indonesia (UMIC)
- Iran (UMIC)
- Iraq (UMIC)
- Jamaica UMIC
- Kenya (LIC_+LDC_+SSA)
- Kiribati (LIC_+LDC_+SSA)
- N. Korea (LIC_+LDC_+SSA)
- Lao PDR (LIC_+LDC_+SSA)
- Lesotho (LIC_+LDC_+SSA)
- Liberia (LIC_+LDC_+SSA)
- Libya (UMIC)
- Madagascar (LIC_+LDC_+SSA)
- Malawi (LIC_+LDC_+SSA)
- Maldives (UMIC)
- Mali (LIC_+LDC_+SSA)
- Marshall islands (UMIC)
- Mauritini (LIC_+LDC_+SSA)
- Mauritius (LIC_+LDC_+SSA)
- Moldova (LMIC)
- Mongolia (LMIC)
- Morocco (LMIC)
- Mozambique (LIC_+LDC_+SSA)
- Myanmar (LIC_+LDC_+SSA)
- Namibia (LIC_+LDC_+SSA)
- Nepal (LIC_+LDC_+SSA)
- Nicaragua (LMIC)
- Niger (LIC_+LDC_+SSA)
- Nigeria (LIC_+LDC_+SSA)
- Pakistan (LMIC)
- Papau New guinea (LMIC)
- Paraguay (UMIC)
- Philippines (LMIC)
- Rwanda (LIC_+LDC_+SSA)
- Samoa (UMIC)
- Sao tome and Principe (LIC_+LDC_+SSA)
- Senegal (LIC_+LDC_+SSA)
- Seychelles (LIC_+LDC_+SSA)
- Sierra Leone (LIC_+LDC_+SSA)
- Solomon islands (LIC_+LDC_+SSA)
- Somalia (LIC_+LDC_+SSA)
- South Africa (LIC_+LDC_+SSA)
- South Sudan (LIC_+LDC_+SSA)
- Sri lanka (LMIC)
- St vincent and the grenadines (UMIC)
- St.Lucia (UMIC)
- Suriname (UMIC)
- Syrian Arab republic (LIC_+LDC_+SSA)
- Tajakistan (LIC_+LDC_+SSA)
- Tanzania (LIC_+LDC_+SSA)
- Timor (LIC_+LDC_+SSA)
- Tonge (UMIC)
- Tunisia (LMIC)
- Tuvalu (LIC_+LDC_+SSA)
- Uzbekistan (LMIC)
- Vanuate (LIC_+LDC_+SSA)
- Venezuela (UMIC)
- Vietnam (LMIC)
- Yemen (LIC_+LDC_+SSA)
- Zambia (LIC_+LDC_+SSA)
- Zimbabwe (LIC_+LDC_+SSA)